- Home
- Greenchemistry
- Research
Research Projects
Daniel Dowling
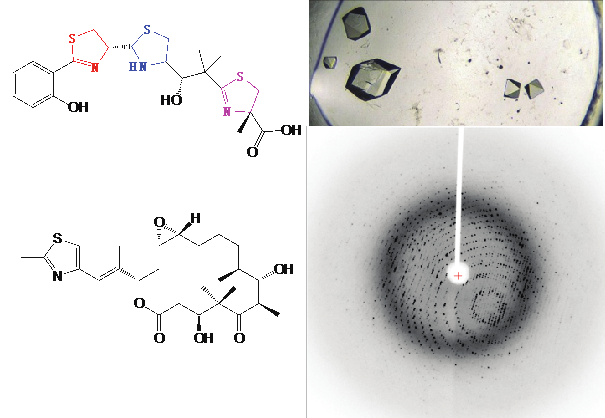
Research in the Dowling lab is at the interface of chemistry and biology. We are interested in utilizing the structural technique of X-ray crystallography and enzymatic assays to elucidate the function and mechanism of different biological systems. One field of particular interest for green chemistry is natural product biosynthesis. By increasing our understanding of how enzymes perform challenging chemical transformations at the molecular level, we aim to develop systems that can be further manipulated for benefits that include biocatalysis and natural product engineering.
Select Publications
Dowling, D.P. et al. Radical SAM enzyme QueE defines a new minimal core fold and metal-dependent mechanism. Nat. Chem. Biol. 10, 106–112 (2014).
Dowling, D.P., Croft, A.K. & Drennan, C.L. Radical use of Rossmann and TIM barrel architectures for controlling coenzyme B12 chemistry. Annu. Rev. Biophys. 41, 403–427 (2012).
Dowling, D.P., Vey, J.L., Croft, A.K. & Drennan, C.L. Structural diversity in the AdoMet radical enzyme superfamily. Biochim. Biophys. Acta 1824, 1178–1195 (2012).
Jason Evans
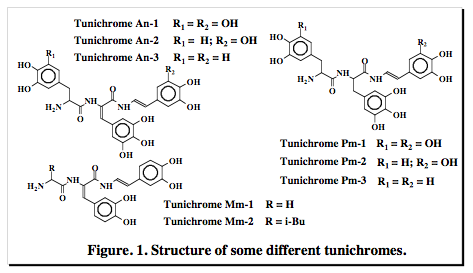
Two problems associated with the arsenal of antibiotics commonly used to treat infections are the evolutionary development of resistance and persistence in our environment, which presents risks to aquatic ecosystems, as well as, to human health. The Evans laboratory is interested in developing a strategy for discovering a new greener class of antibiotics, based on tunichromes extracted and isolated from anthropods.
Michelle Foster
The Foster Group does Surface Chemistry and Experimental Physical Chemistry. We use optical spectroscopies, such as diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), and scanning probe microscopies, such as atomic force microscopy (AFM), and combination techniques, such as a Raman microscope spectrometer, to study chemical reactions and processes happening at complex interfaces. Green Chemistry: Adsorption studies on metal oxide nanoparticles to help explain heterogeneous catalytic processes; Mechanistic studies on solid acid catalysts. Materials Science: Nanomechanical properties of novel core-shell nano/micro-spherical particles; Physical properties of novel liquid-metal systems. Alternative Energy Processes: Adsorption processes in dye-sensitized solar cells; Modification and optimization of activated carbon. Biological Chemistry: Investigate the morphology of amyloid fibrils; Visualize other biological macromolecules.
Select publications:
Carbon surface functionalities and SEI formation during Li intercalation. John Collins, Gerald Gourdin, Michelle Foster, Deyang Qu. Carbon, 92, 193-244, 2015. (DOI: 10.1016/j.carbon.2015.04.007)
Partial graphitization of activated carbon by surface acidification. John Collins, Dong Zheng, Tue Ngo, Deyang Qu, Michelle Foster. Carbon, 79, 500-517, 2014. (DOI: 10.1016/j.carbon.2014.08.009)
Spectroscopic compositional analysis of electrolyte during initial SEI layer formation. Gerald Gourdin, John Collins, Dong Zheng, Michelle Foster, Deyang Qu. Journal of Physical Chemistry C, 118(31) 17383-17394, 2014. (DOI: 10.1021/jp50418b)
Design, synthesis and biological activity of multifunctional a,b-unsaturated carbonyl scaffolds for Alzheimer’s disease. Seema Bag, Sanjukta Ghosh, Rekja Tulsan, Abha Sood, Weihong Zhou, Christine Schifone, Michelle Foster, Harry LeVine, Bela Török, Marianna Török. Bioorganic & Medicinal Chemistry Letters, 23(9) 2614-2618, 2013. (DOI: 10.1016/j.bmcl.2013.02.103)
Spectroscopic investigations of sequential nitric acid treatments on granulated activated carbon: Effects of surface oxygen groups on π-density. John Collins, Tue Ngo, Deyang Qu and Michelle Foster. Carbon, 57, 174-183, 2013. (DOI: 10.1016/j.carbon.2013.01.061)
Jason Green
Professor Green's research focuses on solving complex chemical problems with statistical mechanics. Problems of interest include self-assembly, the mixing of complex liquids, and combustion. A significant effort is to develop theoretical and computational methodologies that will enable more energy and atom efficient chemistry, and to improve our understanding of the reaction environments underlying chemical and biological reactions.
Selected publications:
Reactive symbol sequences for a model of hydrogen combustion Mohammad Alaghemandi, Jason R. Green Physical Chemistry Chemical Physics 2016 18(4), p. 2810-2817
Order and disorder in irreversible decay processes Jonathan W. Nichols, Shane W. Flynn, Jason R. Green
The Journal of Chemical Physics 2015 142(6) p. 064113
Measuring disorder in irreversible decay processes Shane W. Flynn, Helen C. Zhao, Jason R. Green
The Journal of Chemical Physics 2014 141(10) p. 104107
A relationship between dynamical entropy and energy dissipation far from thermodynamic equilibrium
Jason R. Green, Anthony B. Costa, Bartosz A. Grzybowski, Igal Szleifer
Proc. Natl. Acad. Sci. USA 2013 110(41) p. 16339-16343
Jonathan Rochford
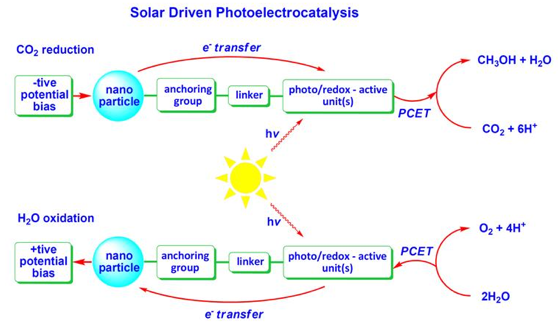
Green chemistry projects in Prof. Rochford's research group are focused on the development of nanoparticle-molecule architectures towards applications in solar driven catalysis. Fundamental processes such as proton coupled electron-transfer (PCET) and charge-separation in the solid state are investigated. Major interests include CO2 reduction and water oxidation catalysis.
Selected Publications:
“Characterization of Redox States of Ru(OH2)(Q)(tpy)2+ (Q = 3,5-di-tert-butyl-1,2- benzoquinone, tpy = 2,2’:6’,2”-terpyridine) and Related Species through Experimental and Theoretical Studies” Tsai M.-K.; Rochford J.; Polyansky D. E.; Tanaka K.; Fujita E.; Muckerman J. T., Inorg. Chem., 2009, 48, 4372.
"Photoelectrochemical Behavior of Polychelate Porphyrin Chromophores and Titanium Dioxide Nanotube Arrays for Dye-Sensitized Solar Cells" de Tacconi N. R.; Chanmanee W.; Rajeshwar K.; Rochford J.; Galoppini E., J. Phys. Chem. C, 2009, 113, 2926.
"Zn (II) Tetraarylporphyrins Anchored to TiO2, ZnO and ZrO2 Nanoparticle Films Through Rigid-Rod Linkers" Rochford J.; Gallopini E. Langmuir, 2008, 24, 5366.
"Tetrachelate Porphyrin Chromophores for Metal Oxide Semiconductor Sensitization: The effect of Spacer Length and Anchoring Group Position" Rochford J.; Chu D.; Hagfeldt A.; Galoppini, E. J. Am. Chem. Soc., 2007, 129, 4655.
Fast Electron Transport in MOCVD-Grown Dye-Sensitized ZnO Nanorod Solar Cells" Galoppini E.; Rochford J.; Chen H.; Saraf G.; Lu Y.; Hagfeldt A.; Boschloo G. J. Phys. Chem. B, 2006, 110, 16159.
Niya Sa
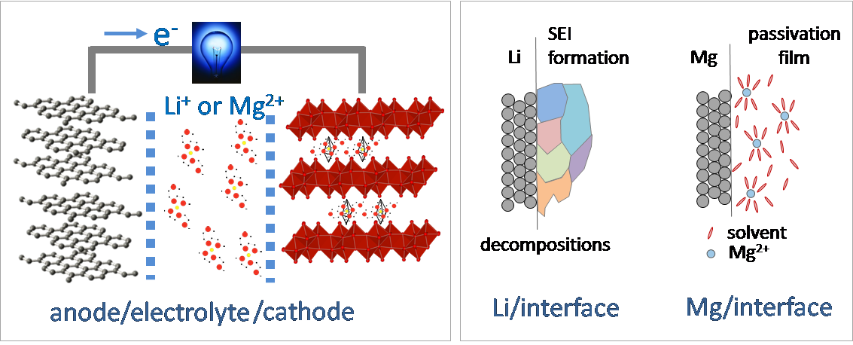
Research in Sa’s lab aims to probe and elucidate in-operando chemical processes at the interfaces of high performance, low cost energy storage materials. One particular research interest is to look for multivalent rechargeable ion batteries that can potentially provide a higher energy density at a lower cost. One broad impacts of such research relating to Green Chemistry includes promoting the adoption of electric vehicles (EV) to reduce or eliminates the need for imported oil and gas.
Selected recent publications/patents:
Sa, N.; Kinnibrugh, T.; Wang, H,; Gautam, G.; Chapman, K.; Vaughey, J.; Key, B.; Fister, T.; Freeland, J.; Proffit, D.; Chupas, P.; Ceder, G.; Burrell, A., Structural Evolution of Reversible Mg Insertion into a Bilayer Structure of V2O5·nH2O Xerogel Material. Chem. Mater, 2016, 28(9), 2962-2969
[url=http://pubs.acs.org/doi/abs/10.1021/acs.chemmater.6b00026]http://pubs.acs.org/doi/abs/10.1021/acs.chemmater.6b00026[/url]
Sa, N.; Wang, H,; Proffit, D.; Lipson, A.; Key, B.; Liu, M.; Feng, Z.; Fister, T.; Ren, Y.; Sun, C.; Vaughey, J.; Fenter, P; Persson, K.; Burrell, A., Is Alpha-V2O5 a Cathode Material for Mg Insertion Batteries? J. Power. Sources, 2016, 323, 44-50
Link: [url=http://www.sciencedirect.com/science/article/pii/S0378775316305651]http://www.sciencedirect.com/science/article/pii/S0378775316305651[/url]
Sa, N.; Pan, B.; Saha, A.; Hubaud, A.; Vaughey, J.; Baker, L.; Burrell, A., Role of Chloride for a Simple, Non-Grignard Mg Electrolyte in Ether Based Solvents. ACS Appl. Mater. Interfaces, 2016, 8(25), 16002-16008
Link: [url=http://pubs.acs.org/doi/abs/10.1021/acsami.6b03193?src=recsys&journalCode=aamick]http://pubs.acs.org/doi/abs/10.1021/acsami.6b03193?src=recsys&journalCode=aamick[/url]
Sa, N.; Rajput, N.; Wang, H.; Baris, K.; Persson, K.; Burrell, A.; Vaughey, J., A Concentration Dependent Study of a Conventional Magnesium bis(trifluoromethane sulfonyl)imide Diglyme Electrolyte, RSC. Advances, 2016, 6, 113663-113670
Link: [url=http://pubs.rsc.org/-/content/articlelanding/2016/ra/c6ra22816j/unauth#!divAbstract]http://pubs.rsc.org/-/content/articlelanding/2016/ra/c6ra22816j/unauth#!divAbstract[/url]
Patent: Electrolytes for magnesium electrochemical cells, US 9698448 B2
Link: [url=http://www.google.com.pg/patents/US20160308248]http://www.google.com.pg/patents/US20160308248[/url]
Neil Reilly
Prof. Reilly’s research aims to identify reactive molecules known or thought to be involved in the early stages of combustion and pyrolysis of biomass and fossil fuels. Particular targets of interest are radicals that can lead to the formation of environmentally harmful byproducts such as polycyclic aromatic hydrocarbons. Transient species are produced from stable fuel precursors in a discharge or thermal cracking source and isolated in a vacuum chamber to “freeze out” further chemistry. Subsequently, laser-based mass-selective and fluorescence spectroscopies are employed to interrogate the products and determine their electronic and molecular structures, which are vital inputs for validating and generating models of combustion chemistry.
Selected publications:
“Jet-Cooled Spectroscopy of the a-methylbenzyl radical: probing the state-dependent effects of methyl rocking against a radical site", Kidwell, N. M., Reilly, N. J., Nebgen, B., Mehta-Hurt, D. N., Hoehn, R. D., Kokkin, D. L., McCarthy, M. C., Slipchenko, L. V., Zwier, T. S., Journal of Physical Chemistry A, 2013, 117(50):13465-80.
“Spectroscopic Identification of the Resonance- Stabilized cis- and trans-1-Vinylpropargyl Radicals", Reilly, N. J., Nakajima, M., Troy, T. P., Chalyavi, N., Duncan, K. A., Nauta, K., Kable, S. H., Schmidt, T. W., Journal of the American Chemical Society, 2009, 131, (37), 13423-13429.
“Laser-induced fluorescence and dispersed fluorescence spectroscopy of jet-cooled 1-phenylpropargyl radical", Reilly, N. J.; Nakajima, M.; Gibson, B. A.; Schmidt, T. W., Kable, S. H., Journal of Chemical Physics, 2009, 130, (14), 144313.
“Spectroscopic observation of the resonance-stabilized 1-phenylpropargyl radical", Reilly, N. J.; Kokkin, D. L.; Nakajima, M.; Nauta, K.; Kable, S. H.; Schmidt, T. W., Journal of the American Chemical Society, 2008, 130, (10), 3137-3142.
Hannah Sevian
The Sevian Research Group studies how students develop conceptual understanding of chemistry and how research-based instruction and materials impact student learning, particularly in chemistry; how scientists and science teachers can communicate science more effectively; and how to improve the pipeline for students in K-12 and higher education, particularly in urban schools, to remain in science career pathways.
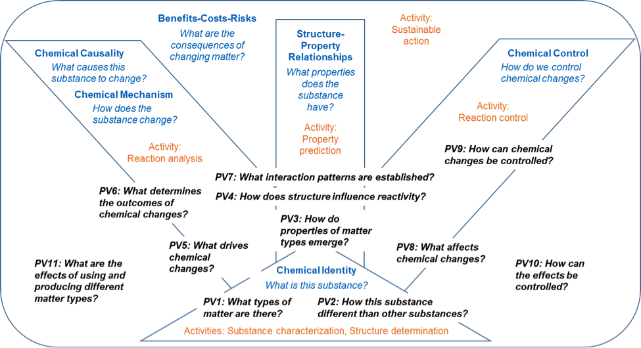
Chemical thinking framework (DOI: 10.1039/C3RP00111C)
Selected publications:
Banks, B.; Clinchot, M.; Cullipher, S.; Huie, R.; Lambertz, J.; Lewis, R.; Ngai, C.; Sevian, H.; Szteinberg, G.; Talanquer, V.; Weinrich. M. Uncovering chemical thinking in students' decision making: A fuel-choice scenario. J. Chem. Educ. 2015, 92(10), 1610-1618.
Cullipher, S.; Sevian, H.; Talanquer. V. Reasoning about benefits, costs, and risks of chemical substances: Mapping different levels of sophistication. Chem. Educ. Res. Pract. 2015, 16, 377-392.
Ngai, C.; Sevian, H.; Talanquer, V. What is this substance? What makes it different? Mapping progression in students' assumptions about chemical identity. Intl. J. Sci. Educ., 2014, 36(14), 2438-2461.
Sevian, H.; Bernholt, S.; Szteinberg, G.; Auguste, S.; Pérez, L.C. Use of representation mapping to capture abstraction in problem solving in different courses in chemistry. Chem. Educ. Res. Pract. 2015, 16, 429-446.
Sevian, H.; Talanquer, V. Rethinking chemistry: A learning progression on chemical thinking. Chem. Educ. Res. Pract., 2014, 15(1), 10-23.
Wei Zhang
Green chemistry research projects in Prof. Zhang's group are carried out through the development of fluorous technologies for organic and medicinal chemistry applications. The green technologies include chromatography-free separations, metal-free and recyclable organocatalysts, atom/step economic one-pot and multicomponent reactions.
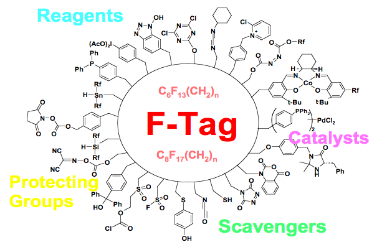
Selected publications:
Huang, X.; Pham, K.; Yi, W.; Zhang, X.; Clamens C.; Hyatt, J. P.; Jasinsk, J. P.; Tayvah, U.; Zhang, W. “Recyclable Organocatalyst-promoted One-pot Asymmetric Synthesis of Spirooxindoles Bearing Multiple Stereogenic Centers” Adv. Synth. Catal. 2015, 357, 3820-3824.
McKeown, M.; Shaw, D.; Fu, H.; Liu, S.; Xu, X.; Marineau, J.; Huang, Y.; Zhang, X.; Buckley, D.; Lin, C.; Kadam, A.; Zhang, Z.; Blacklow, S.; Qi, J.; Zhang, W.; Bradner, J. “Biased multicomponent reactions to develop novel bromodomain inhibitors” J. Med. Chem. 2014, 57, 9019-9027.
Zhang, W., Cue, B. Eds. Green Techniques for Organic Synthesis and Medicinal Chemistry; Wiley, 2012.
Zhang, W. "Green Chemistry Aspects of Fluorous Techniques - Opportunities and Challenges for Small-Scale Organic Synthesis" Critical review article, Green Chem. 2009, 11, 911-920.
Zhang, W. "Fluorous Linker-Facilitated Chemical Synthesis" Chem. Rev. 2009, 109, 749-795.